Search:
Page Number:
Copper or Carbon?Skills Reference 2 Skills you Will Use
Safety
Caution Use caution when working around the hotplate, it can cause burns if it comes in contact with your skin. Wear goggles to protect your eyes from the materials used in this lab. Two of the main classifications of the periodic table are metals and non-metals.
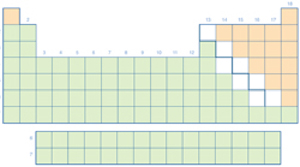
However, what characteristics of elements result in their classification as metal or non-metal? In this activity, you will examine copper and carbon to determine what characteristics are attributable to metals and non-metals. QuestionWhat are the characteristics of metals and non-metals? Materials and Equipment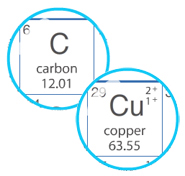
Dry LabA "dry lab" activity includes collected data and/or a video solution for your convenience. You can simply watch the following video and use the provided data, or if you wish to perform this lab for yourself, follow the procedure steps 1 through 12 described in the video. The same steps are included in written form in the documents available for download on the bottom of this page. Analyzing and Interpreting
Forming Conclusions
|