Search:
Page Number:
Shape Up!Skills Reference 2 Skills you Will Use
Safety
Caution In this lab you will be using hot water. Use a thick glove or gloves to protect yourself from burns. In an ionic compound, positive and negative ions attract each other, with all the positive ions attracted to all of the negative ions, forming ionic bonds. Ionic bonds cause ions to group together in an alternating pattern of charged particles called a crystal arrangement.
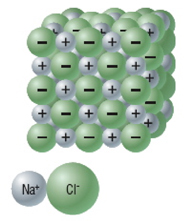
In this activity, you will investigate the formation of salt crystals to find evidence of ionic compound crystal arrangement. QuestionWhat is the structure of sodium chloride crystals? Materials and Equipment
Dry LabA "dry lab" activity includes collected data and/or a video solution for your convenience. You can simply watch the following video and use the provided data, or if you wish to perform this lab for yourself, follow the procedure steps 1 through 8 described in the video. The same steps are included in written form in the documents available for download on the bottom of this page. Analyzing and Interpreting
Forming Conclusions
|